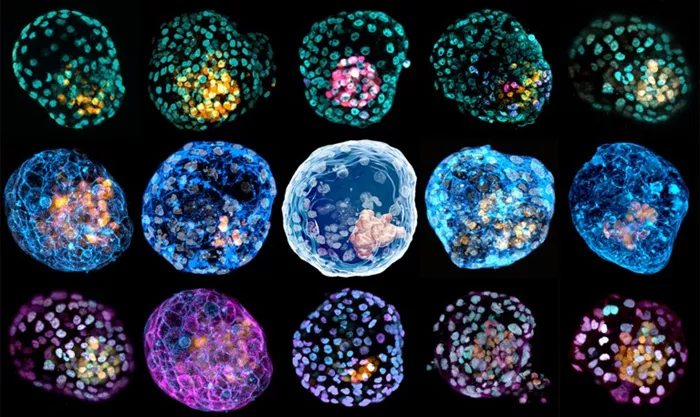
The earliest days of human development—right after sperm meets egg—remain one of biology’s greatest mysteries.
Understanding how a single cell transforms into a complex organism is crucial for unraveling fertility challenges, developmental disorders, and genetic conditions. However, for mammals, this intricate process occurs within the protective environment of the uterus, making direct observation difficult. Scientists have long struggled to pinpoint what can go wrong in these critical early stages, especially when genetic factors or environmental influences disrupt embryo formation.
Now, researchers at UC Santa Cruz have developed lab-grown cellular models that mimic the first few days after fertilization without using actual embryos. By employing CRISPR-based genetic tools, they have created “programmable” embryo-like structures—embryoids—that offer a new window into early development. Their findings, published in Cell Stem Cell, could reshape how scientists study reproductive health and early-stage diseases.
Engineering Embryo Models for Scientific Discovery
Assistant Professor Ali Shariati, a specialist in biomolecular engineering, explains that recreating natural developmental processes in the lab allows scientists to study them in ways that would be impossible using traditional embryo research.
“We want to understand how cells organize into an embryo-like structure and what happens when developmental conditions go awry,” says Shariati.
The team, led by UCSC postdoctoral scholar Gerrald Lodewijk and biomolecular engineering alumna Sayaka Kozuki, worked with mouse stem cells—blank slates capable of transforming into various cell types, including brain and gut cells. Using an advanced CRISPR tool called an epigenome editor, which modifies gene expression without altering DNA sequences, they guided these stem cells into self-organizing embryo-like formations.
This approach allowed researchers to control gene activation, triggering the formation of essential cell types required for early development. Unlike traditional chemical methods, which produce individual cell types separately, this technique allowed different cell types to co-develop simultaneously—closely resembling the natural embryo formation process.
How Cells ‘Self-Organize’ in Early Development
A key breakthrough in this study was observing how stem cells naturally grouped and behaved in ways similar to actual early embryos. In about 80% of cases, the lab-grown cells successfully formed structures mimicking the earliest embryonic stages.
“The way the cells organize themselves and interact is strikingly similar to what we see in natural embryos,” Shariati explains. “They need very little intervention from us—it’s as if they already know what to do.”
One particularly fascinating observation was how the cells exhibited collective movement. Some migrated in a rotational pattern, similar to the coordinated movements of birds in flight, which played a role in shaping embryonic structures. This self-driven behavior could provide insights into how early-stage developmental disorders arise when these cellular processes fail.
‘Programmable’ Models for Studying Genetic Disorders
One of the most promising aspects of these embryo-like models is their programmability. Because CRISPR tools allow precise activation of genes at different stages, scientists can systematically test how various genetic factors contribute to developmental disorders.
“These models give us a more complete picture of early development,” says Lodewijk. “We can analyze how different genes influence cell formation and identify what happens when specific genes are switched on or off.”
By controlling gene activation in a highly targeted manner, researchers can simulate the effects of genetic mutations or environmental conditions that may hinder embryo development. For example, they demonstrated how certain tissues either formed correctly or failed due to specific genetic modifications. This method could be applied to studying congenital defects, reproductive challenges, and genetic disorders.
Future Applications in Fertility and Reproductive Health
Beyond understanding genetic conditions, this research could help address one of the biggest hurdles in human reproduction—why so many embryos fail to implant or properly develop in early pregnancy. Compared to other mammals, humans face higher rates of early developmental failure, and scientists are still working to understand why.
“By studying these bottlenecks in early development, we could potentially improve fertility treatments and enhance reproductive success,” says Shariati.
The researchers also hope to extend this model to study embryo development in other species. Since the technique does not rely on actual embryos, it offers an ethical and scalable way to explore reproductive biology across various organisms.
Ultimately, these embryo models could transform how scientists investigate early human development, shedding light on fertility, genetic disorders, and regenerative medicine—all without the need for traditional embryo experimentation.
Related topics:
- Experimental Drug Shows Promise in Delaying Dementia Onset for Genetic Alzheimer’s Patients
- Benadryl Liquid Elixir Recall: Potential Poisoning Risk for Children Leads to Urgent Action
- Health Authorities Warn Against Unproven Cancer Treatments Sold Online